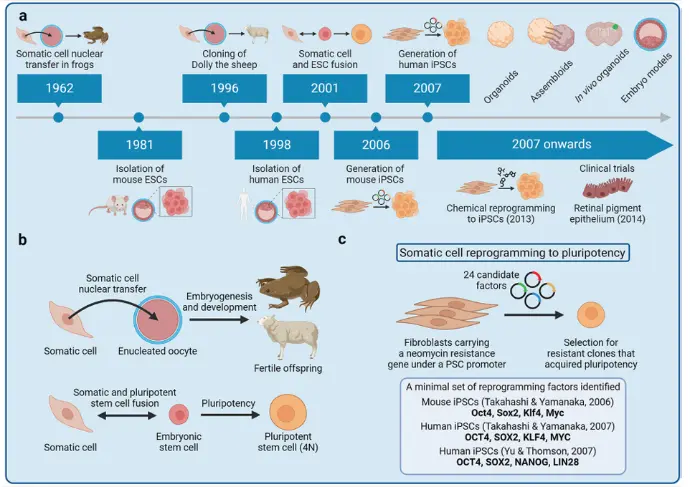
Molecular mechanisms of somatic cell reprogramming to iPSCs involve the conversion of differentiated somatic cells into a pluripotent state through the forced expression of specific transcription factors classically OCT4, SOX2, KLF4, and c-MYC (OSKM) as first demonstrated by Shinya Yamanaka in 2006. These factors act by remodeling the epigenetic landscape of the cell, silencing lineage-specific genes while activating pluripotency-associated networks. During reprogramming, chromatin undergoes extensive modifications: DNA methylation at promoters of pluripotency genes (such as NANOG and LIN28) is erased, histone acetylation increases, and repressive histone marks (like H3K9me3 and H3K27me3) are removed. This epigenetic remodeling facilitates the reactivation of endogenous pluripotency circuits that stabilize the iPSC state. Additionally, non-coding RNAs, signaling pathways (Wnt/β-catenin, TGF-β, and MAPK), and metabolic reprogramming play crucial roles cells shift from oxidative phosphorylation toward glycolysis, mimicking embryonic stem cell metabolism. Recent studies (2023–2025) have uncovered the involvement of chromatin remodelers such as BRG1, epigenetic modifiers like TET1/2, and RNA-binding proteins in fine-tuning the efficiency and fidelity of reprogramming. These discoveries enhance our understanding of how cell identity can be reset and are paving the way for safer, more controlled iPSC generation for regenerative medicine and toxicology applications