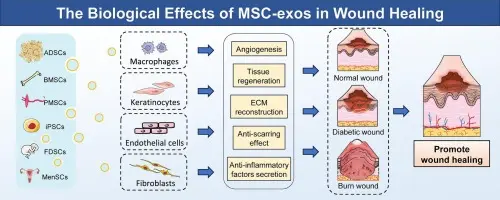
Exosomes from human umbilical cord mesenchymal stromal/stem cells (hUC-MSC-Exos) are a fast-growing, promising cell-free therapeutic platform. They show strong preclinical evidence for regenerative and immunomodulatory effects (wound healing, kidney disease, cartilage repair, liver regeneration, hair growth), are being engineered for targeted delivery, and are entering early-stage clinical testing but translation faces manufacturing, potency-assay, safety and regulatory hurdles that the field is actively addressing.
Why researchers favor umbilical-cord-derived MSC exosomes
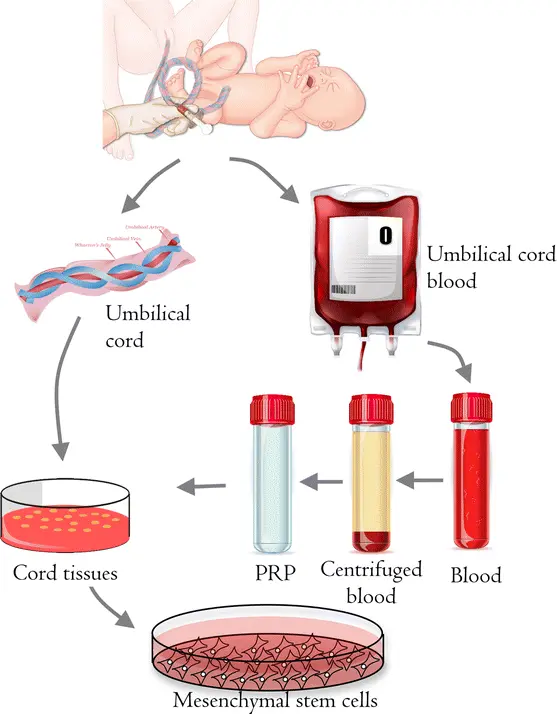
Umbilical cord (Wharton’s jelly) is an attractive MSC source because it’s abundant, non-invasive to collect, yields cells with strong proliferative potential, and tends to produce exosomes with robust regenerative and immunomodulatory cargo (miRNAs, proteins) compared with aged adult tissue sources. This makes hUC-MSC exosomes appealing for scalable, off-the-shelf therapeutics and avoids some limitations of donor-age variation seen in bone-marrow MSCs.
Basic properties & characterization of hUC-MSC-Exos
Isolation & physical features
- Exosomes are typically isolated via ultracentrifugation, size-exclusion, precipitation kits, etc. For example, one study of hUC-MSC-Exos characterised vesicles of ~50-150 nm diameter, positive for canonical exosome markers CD63, CD81, TSG101.
- Morphologically, TEM (transmission electron microscopy) shows spherical or cup-shaped vesicles with a bilayer membrane.
- Nanoparticle tracking analysis (NTA) or dynamic light scattering (DLS) are commonly used to quantify size distribution and concentration. (E.g., see the oxalate-kidney study)
Cargo and functional potential
- The cargo of hUC-MSC-Exos includes: microRNAs (miRNAs), mRNAs, proteins (including growth factors, signalling modulators), lipids. Many of these cargo elements mediate the biological activity of the exosomes.
- Proteomic studies show that hUC-MSC exosomes have distinct protein profiles. For instance, one study compared exosome mimetic vesicles (EMVs) vs “natural” exosomes from hUC-MSCs and conducted comprehensive proteomics to chart differences in content and yield.
Mechanisms of cell–cell communication
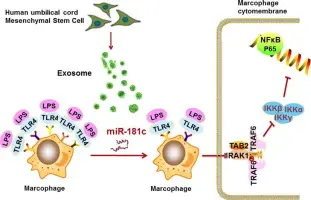
- Recipient cells can internalize exosomes (via endocytosis, membrane fusion). For example, hUC-MSC-Exos were taken up by macrophages and influenced their phenotype.
- Once internalized, exosome cargo can modulate signalling pathways in recipient cells: e.g., altering gene expression, miRNA-mediated target silencing, modifying inflammatory signalling, promoting angiogenesis or anti-apoptosis.
- Example: In an IL-6 stimulated model, hUC-MSC-Exos inhibited macrophage activation and inflammatory cytokine release.
Key therapeutic applications & evidence from models
Below are major application areas where hUC-MSC-Exos show promise with supporting preclinical (and some emerging clinical) data.

Musculoskeletal / cartilage / osteoarthritis
- In a rat model of osteoarthritis (OA), hUC-MSC-Exos promoted cartilage regeneration, reduced pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) in synovial fluid, reduced expression of degradative enzymes (MMP-13, ADAMTS-5), and increased type II collagen expression.
- Mechanistically, they also influenced macrophage polarization (shifting from M1 to M2-like phenotype) thus reducing inflammation and promoting regenerative environment.
- A more recent study reported translation into early clinical research: intra-articular injection of hUC-MSC-Exos in knee osteoarthritis patients showed safety and some efficacy signals (improved clinical scores, MRI changes) in a small cohort.

Liver disease / non-alcoholic steatohepatitis (NASH)
- In a mouse model of NASH (methionine- and choline-deficient diet induced), injection of hUC-MSC-Exos improved liver damage, body weight loss, reduced inflammatory cytokines, and reversed down-regulation of PPARα in hepatocytes.
- This suggests potential for metabolic-liver disease indications where inflammation, fibrosis and metabolic dysregulation are central.

Metabolic / adipocyte / insulin resistance
- A study treated insulin-resistant human adipocytes with hUC-MSC-Exos and found improved insulin-stimulated glucose uptake, reduced leptin levels, upregulated SIRT1 and IRS-1 mRNA indicating enhanced insulin sensitivity.
- This indicates potential use in metabolic disorders (type-2 diabetes, obesity-related insulin resistance) though still early.

Other applications (emerging)
- Some studies show effects in wound healing/migration of endometrial glandular epithelial cells: hUC-MSC-Exos enhanced migration and altered EMT markers (N-cadherin, vimentin, E-cadherin) in endometrial models.
- Skin/dermal repair, neuro-regeneration and other organ repair roles are being explored: recent reviews highlight potential for skin (UV-damage), nerve injury, etc.
Trends & evolving themes in the field
Shift toward exosome (“cell-free”) therapies
Given the challenges of cell therapies (engraftment inefficiency, cost, safety concerns), there is increasing interest in moving toward exosome-based therapeutics. Many investigators consider hUC-MSC-Exos as a next-generation therapeutic product. Evidence in numerous disease models supports this shift.
Engineering & functional enhancement
Researchers are not just using “native” exosomes, but engineering them in several ways:
Preconditioning MSCs (e.g., with IL-6) to increase exosome yield and modify cargo. For example, IL-6 stimulation of hUC-MSCs increased exosome secretion ~3× and enhanced anti-inflammatory effects in macrophages.
Loading exosomes with specific miRNAs or therapeutic molecules. Example: hUC-MSC-Exos delivering miR-26a-5p inhibited nucleus pulposus cell pyroptosis via METTL14/NLRP3 pathway.
Targeting modifications: surface ligands, targeting peptides, future directions include exosome “tropism” improvement. Recent review highlights this path.
Manufacturing, characterization, yield improvement
A key limitation of exosome therapies is low natural yield per cell and challenges in large scale purification. Studies comparing exosome mimetic vesicles (EMVs) vs traditional exosomes explore alternative production routes.
Standardization efforts: defining exosome markers, size/distribution, particle/protein ratio metrics, potency assays. The field increasingly emphasises rigorous characterisation.
Regulatory readiness: The literature now includes discussions of translation-ready processes (GMP, closed systems, reproducible production, validated quality control).
Immunomodulation and macrophage polarization
One theme emerging strongly: hUC-MSC-Exos can influence immune cell behaviour, particularly macrophages (shifting from pro-inflammatory to anti-inflammatory phenotypes). This is significant because many chronic diseases have an inflammatory or immune dysregulation component. For example, in cartilage repair studies they modulate macrophage polarization.
Moving toward clinical translation
While most data remain preclinical, some early human/clinical studies are now appearing (e.g., for osteoarthritis) showing favorable safety profiles and preliminary efficacy signals.
Also, mapping studies of research output show rapidly increasing publications in the area of hUC-MSC exosomes. (Though I didn’t pull a specific bibliometric here, the review by Yaghoubia et al. (2019) shows early growth.