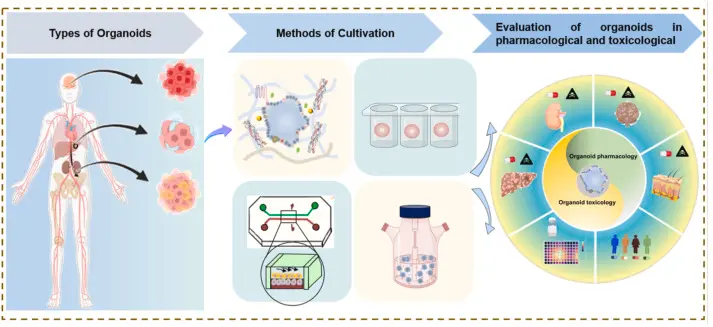
The field of toxicology is undergoing a transformation. Traditional approaches such as 2-dimensional cell culture and animal models have served us faithfully for decades, but they also carry significant limitations when it comes to predicting how humans will respond to chemicals, drugs or environmental toxicants. Enter the era of stem cell-derived and organoid-based in vitro models: 3-dimensional, human-relevant systems that recapitulate key aspects of tissue architecture, cellular diversity and functional behaviour. These “mini-organs in a dish” hold great promise for safer, more predictive toxicology.
Stem cells + differentiation
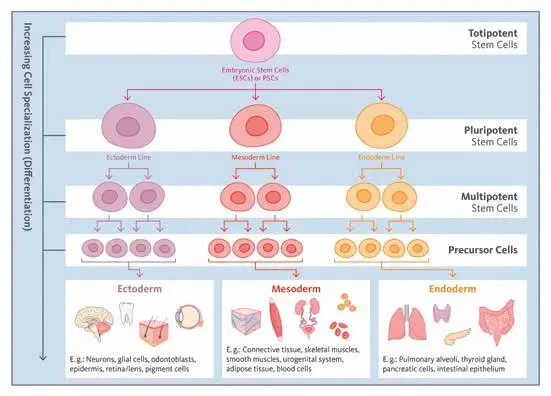
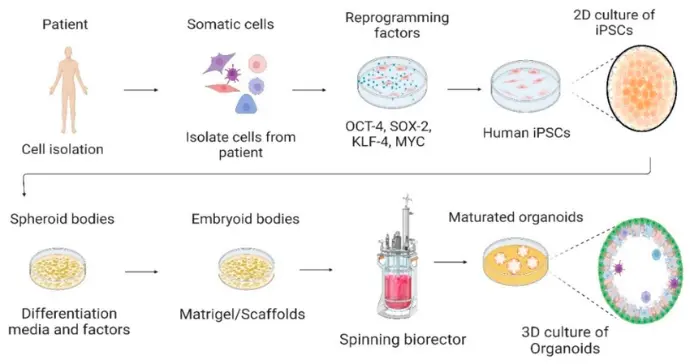
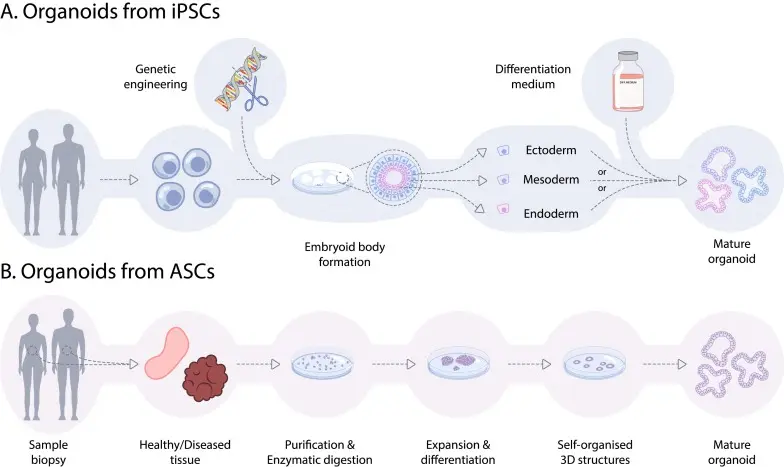
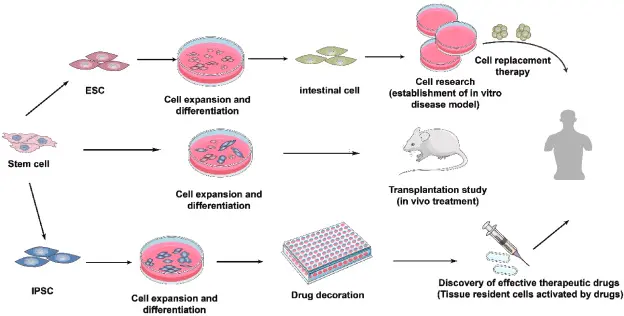
“Stem cells” for our purposes include human pluripotent stem cells (hPSCs), which encompass embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). These cells can differentiate into a wide variety of cell types. Researchers can direct them down specific lineages neuronal, hepatic, cardiac, intestinal, etc. to create relevant cell populations for toxicology.
Organoids
An organoid is a three-dimensional culture generated from stem cells (or adult stem/progenitor cells) that self-organises into a structure mimicking certain aspects of an organ: e.g., multiple cell types, spatial arrangement, functional features (such as metabolism, barrier function or signalling). They exist in contrast to simple monolayer cell cultures.
For example: liver organoids that express drug-metabolising enzymes; intestinal organoids that recreate epithelial barrier and transport functions; neural organoids that model brain development and responses. Recent review work highlights such models for gut and liver in xenobiotic toxicology.

Why call it biology in a dish?

Because these systems bring human-derived tissue functionality into an in vitro format: cells with the donor’s genetics, tissue context, relevant enzyme expression, architecture and this gives us a closer approximation to human biology than many animal models or simple cell lines.
Why are these models particularly interesting for toxicology?
Human-relevance
Animal models often suffer from species differences: metabolism, enzyme expression, receptor binding all may differ in humans vs rodents. Organoid models derived from human cells bypass many of those differences. For example, the 2024 review on iPSC-derived organoids shows they may better predict human developmental neurotoxicity.
Tissue complexity
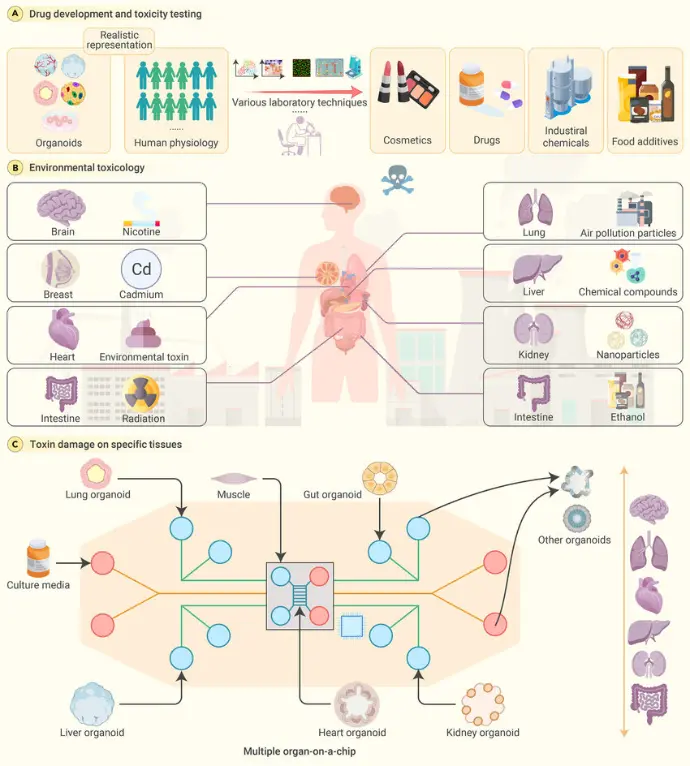
Compared to 2-D cell culture, organoids provide much higher levels of complexity: multiple cell types, three-dimensional architecture, cell-cell and cell-matrix interactions, sometimes defined microenvironments. This allows more realistic responses to toxicants (absorption, metabolism, barrier disruption, interactions). For example, gut organoids show nutrient-absorption and barrier function relevant for chemical exposures.
Mechanistic insight & higher throughput
Organoids can allow mechanistic readouts (gene expression changes, enzyme induction, cellular stress responses, morphological changes) under controlled exposure to toxicants. They also support screening of multiple chemicals/doses. Newer models enable higher throughput than whole-animal studies.
Recent Advances
Here are some of the recent important developments:

Gut & liver organoids in xenobiotic toxicology
A review published March 2025 (“From gut to liver: organoids as platforms for next-generation toxicology assessment vehicles for xenobiotics”) outlines how gut and liver organoids are being implemented.
Key findings:
- Gut organoids derive from stem cells and recreate epithelial cell types (enterocytes, goblet cells, etc.), allowing study of chemical impacts on absorption, barrier integrity, nutrient uptake and xenobiotic interactions.
- Liver organoids express important detoxification enzymes and transporters, allowing more predictive modelling of hepatotoxicity and chemical metabolism.
- Use of these organoids showed exposure to heavy metals (lead, mercury, thallium) and PFAS induced expected toxicity responses.
- The review notes challenges remain (immaturity of some organoids, lack of full vascularisation, heterogeneity) but emphasizes the translational potential.

Advances in liver organoids for toxicity assessment
Another paper (January 2025) titled “Advances in liver organoids: replicating hepatic complexity for toxicity assessment and disease modeling” reports that liver organoids are getting closer to adult liver functionality (metabolism, enzyme activity, bile secretion) though still not fully mature.
It emphasises that these organoids can serve for disease modelling and for toxicology, offering more physiological relevance than 2D hepatocyte cultures.

Neural organoids for developmental neurotoxicity
In November 2024, a study "Human-Induced Pluripotent Stem Cell-Derived Neural Organoids as a Novel In Vitro Platform for Developmental Neurotoxicity Assessment" showed iPSC-derived neural organoids could detect pesticide-induced apoptosis and morphological changes at low doses (e.g., rotenone apoptosis at low dose; chlorpyrifos induced neural progenitor morphological changes).
A February 2025 review “Toxicity assessment using neural organoids: innovative approaches and challenges” further discusses neural organoids in the context of drug/neurotoxicity screening.

Application of stem cells (PSCs) in developmental toxicity
A review in Toxicological Sciences (March 2024) “Pluripotent stem cells for target organ developmental toxicity testing” summarises how human PSC-based 2D and 3D models (including organoids) are used for brain and heart lineage toxicity screening.